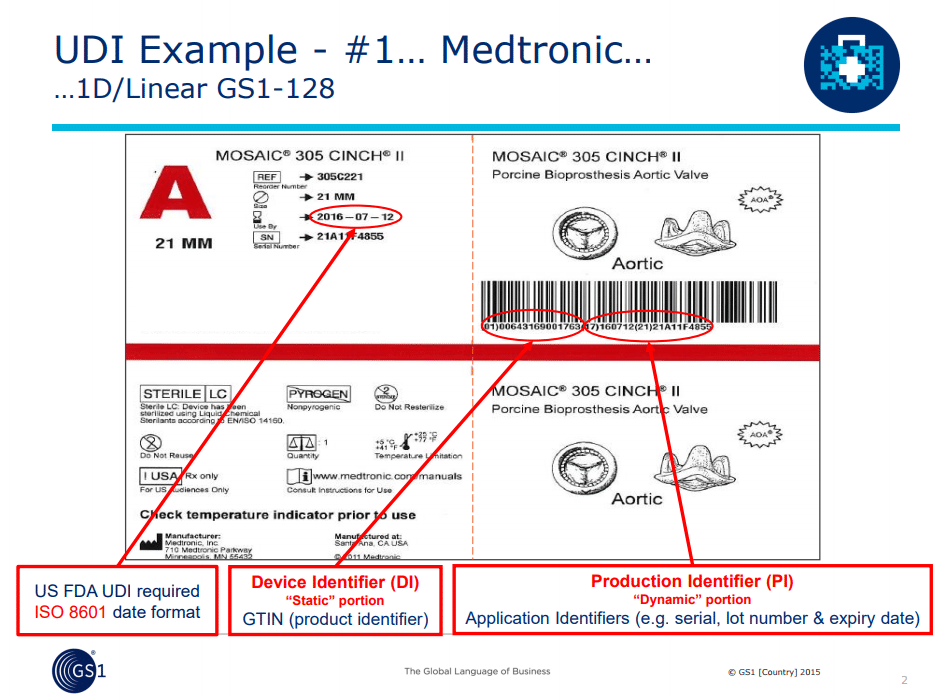
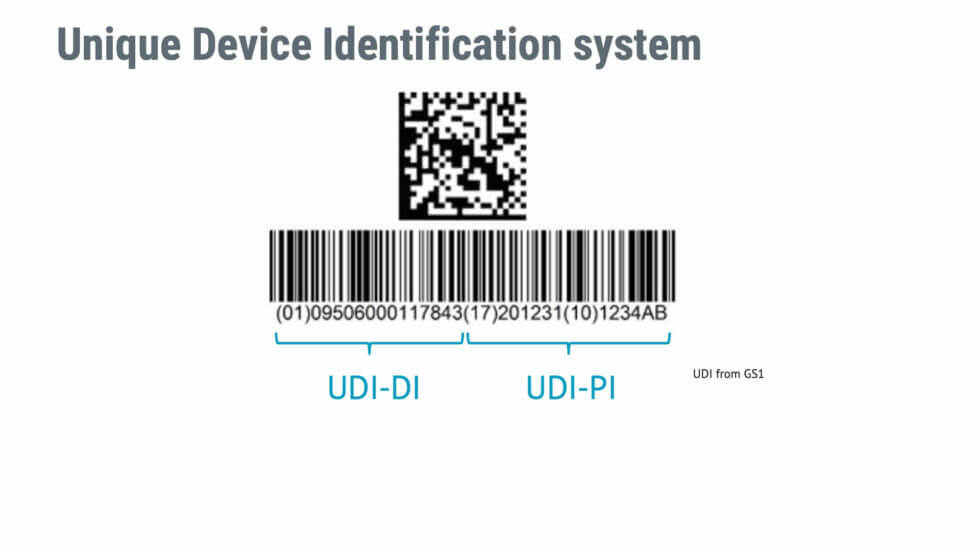
Udi Multiple Catalog Numbers For One Product
Udi Multiple Catalog Numbers For One Product - It offers a quiet, focused space away from the constant noise of digital distractions, allowing for the deep, mindful work that is so often necessary for meaningful progress. It’s crucial to read and understand these licenses to ensure compliance. As artists navigate the blank page, they are confronted with endless possibilities and opportunities for growth. The focus is not on providing exhaustive information, but on creating a feeling, an aura, an invitation into a specific cultural world. It is a journey from uncertainty to clarity. Take advantage of online resources, tutorials, and courses to expand your knowledge. We have seen how a single, well-designed chart can bring strategic clarity to a complex organization, provide the motivational framework for achieving personal fitness goals, structure the path to academic success, and foster harmony in a busy household. It requires a commitment to intellectual honesty, a promise to represent the data in a way that is faithful to its underlying patterns, not in a way that serves a pre-determined agenda. The instant access means you can start organizing immediately. It is a powerful cognitive tool, deeply rooted in the science of how we learn, remember, and motivate ourselves. 50 This concept posits that the majority of the ink on a chart should be dedicated to representing the data itself, and that non-essential, decorative elements, which Tufte termed "chart junk," should be eliminated. Every element on the chart should serve this central purpose. The journey through an IKEA catalog sample is a journey through a dream home, a series of "aha!" moments where you see a clever solution and think, "I could do that in my place. The technological constraint of designing for a small mobile screen forces you to be ruthless in your prioritization of content. 11 This is further strengthened by the "generation effect," a principle stating that we remember information we create ourselves far better than information we passively consume. A truly effective printable is designed with its physical manifestation in mind from the very first step, making the journey from digital file to tangible printable as seamless as possible. From a simple checklist to complex 3D models, the printable defines our time. It gave me the idea that a chart could be more than just an efficient conveyor of information; it could be a portrait, a poem, a window into the messy, beautiful reality of a human life. Having to design a beautiful and functional website for a small non-profit with almost no budget forces you to be clever, to prioritize features ruthlessly, and to come up with solutions you would never have considered if you had unlimited resources. This guide is built on shared experience, trial and error, and a collective passion for keeping these incredible vehicles on the road without breaking the bank. This multimedia approach was a concerted effort to bridge the sensory gap, to use pixels and light to simulate the experience of physical interaction as closely as possible. We see it in the rise of certifications like Fair Trade, which attempt to make the ethical cost of labor visible to the consumer, guaranteeing that a certain standard of wages and working conditions has been met. After the machine is locked out, open the main cabinet door. This was a utopian vision, grounded in principles of rationality, simplicity, and a belief in universal design principles that could improve society. The customer, in turn, receives a product instantly, with the agency to print it as many times as they wish, on the paper of their choice. A 3D printable file, typically in a format like STL or OBJ, is a digital blueprint that contains the complete geometric data for a physical object. They wanted to see the details, so zoom functionality became essential. Even with the most reliable vehicle, unexpected roadside emergencies can happen. This led me to a crucial distinction in the practice of data visualization: the difference between exploratory and explanatory analysis. I wanted to work on posters, on magazines, on beautiful typography and evocative imagery. A truly honest cost catalog would need to look beyond the purchase and consider the total cost of ownership. The democratization of design through online tools means that anyone, regardless of their artistic skill, can create a professional-quality, psychologically potent printable chart tailored perfectly to their needs. A certain "template aesthetic" emerges, a look that is professional and clean but also generic and lacking in any real personality or point of view. Being prepared can make a significant difference in how you handle an emergency. Instead of struggling with layout, formatting, and ensuring all necessary legal and financial fields are included, they can download a printable invoice template. Things like buttons, navigation menus, form fields, and data tables are designed, built, and coded once, and then they can be used by anyone on the team to assemble new screens and features. Thank you for choosing Aeris. This is a monumental task of both artificial intelligence and user experience design. Many products today are designed with a limited lifespan, built to fail after a certain period of time to encourage the consumer to purchase the latest model. The product is often not a finite physical object, but an intangible, ever-evolving piece of software or a digital service. 2 By using a printable chart for these purposes, you are creating a valuable dataset of your own health, enabling you to make more informed decisions and engage in proactive health management rather than simply reacting to problems as they arise. It is not a public document; it is a private one, a page that was algorithmically generated just for me. Lesson plan templates help teachers organize their curriculum and ensure that all necessary components are included. It is a sample of a utopian vision, a belief that good design, a well-designed environment, could lead to a better, more logical, and more fulfilling life. If the download process itself is very slow or fails before completion, this is almost always due to an unstable internet connection. The social media graphics were a riot of neon colors and bubbly illustrations. 50 Chart junk includes elements like 3D effects, heavy gridlines, unnecessary backgrounds, and ornate frames that clutter the visual field and distract the viewer from the core message of the data. For any student of drawing or painting, this is one of the first and most fundamental exercises they undertake. And beyond the screen, the very definition of what a "chart" can be is dissolving. They are the product of designers who have the patience and foresight to think not just about the immediate project in front of them, but about the long-term health and coherence of the brand or product. It has become the dominant organizational paradigm for almost all large collections of digital content. It would shift the definition of value from a low initial price to a low total cost of ownership over time. The concept of printables has fundamentally changed creative commerce. If pressure is low, the issue may lie with the pump, the pressure relief valve, or an internal leak within the system. Each item is photographed in a slightly surreal, perfectly lit diorama, a miniature world where the toys are always new, the batteries are never dead, and the fun is infinite. We know that choosing it means forgoing a thousand other possibilities. It is also the other things we could have done with that money: the books we could have bought, the meal we could have shared with friends, the donation we could have made to a charity, the amount we could have saved or invested for our future. The world of crafting and hobbies is profoundly reliant on the printable template. This is when I encountered the work of the information designer Giorgia Lupi and her concept of "Data Humanism. In the domain of project management, the Gantt chart is an indispensable tool for visualizing and managing timelines, resources, and dependencies. My first encounter with a data visualization project was, predictably, a disaster. I thought my ideas had to be mine and mine alone, a product of my solitary brilliance. Once created, this personal value chart becomes a powerful decision-making framework. It allows you to see both the whole and the parts at the same time. For the first time, I understood that rules weren't just about restriction. It is a process of observation, imagination, and interpretation, where artists distill the essence of their subjects into lines, shapes, and forms. This makes any type of printable chart an incredibly efficient communication device, capable of conveying complex information at a glance. 6 Unlike a fleeting thought, a chart exists in the real world, serving as a constant visual cue. 33 Before you even begin, it is crucial to set a clear, SMART (Specific, Measurable, Attainable, Relevant, Timely) goal, as this will guide the entire structure of your workout chart. The interior rearview mirror should frame the entire rear window. The infotainment system, located in the center console, is the hub for navigation, entertainment, and vehicle settings. When drawing from life, use a pencil or your thumb to measure and compare different parts of your subject. It’s about cultivating a mindset of curiosity rather than defensiveness. Because these tools are built around the concept of components, design systems, and responsive layouts, they naturally encourage designers to think in a more systematic, modular, and scalable way. This posture ensures you can make steering inputs effectively while maintaining a clear view of the instrument cluster. The creator provides the digital blueprint. A designer can use the components in their design file, and a developer can use the exact same components in their code. Types of Online Templates For those who create printable images, protecting their work is equally important. It is a sample of a new kind of reality, a personalized world where the information we see is no longer a shared landscape but a private reflection of our own data trail. It transformed the text from a simple block of information into a thoughtfully guided reading experience.UDI Labeling (Unique Device Identification) Best Practices
Understanding Basic UDIDI and UDIDI in accordance with EU Regulations
UDI Center NESTcc
UDI for Medical Devices Codes & Examples [Ultimate Guide]
UDI BZTAR
Beginners Guide UDI for Unique Device Identification (EU MDR and IVDR)
2024 EU Guide MDR/IVDR Basic UDIDI Casus Consulting
UDI编码介绍 上海格标企业管理咨询有限公司
Prisym UDI Implementation
PPT Unique Device Identification (UDI) Enabling the Transformation
UDI for Medical Devices Codes & Examples [Ultimate Guide]
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR)
PPT UDI From Regulation to Value PowerPoint Presentation, free
Solutions for Medical Devices SEA Vision
EU UDI Requirements, Definition, and Guidance Unique Device Identifier
UDI for Medical Devices Codes & Examples [Ultimate Guide]
What Is a UDI? Essential Guide for Medical Devices
FDA Unique Device Identification (UDI) Overview
The ultimate guide to the China NMPA UDI system and database
Ultimate Guide to UDI for Medical Devices
Stepbystep guide for UDIunder the EU MDR and IVDR
UDI Labeling (Unique Device Identification) Best Practices Lexis
PPT Unique Device Identification Capturing, Exchanging and Accessing
What is a UDI barcode?
UDI requirements for medical device manufacturers in the EU Medical
想知道啥是 UDI?MDR 有什麼規定嗎?來看這篇文章吧! 超強法規筆記本
12 Steps for Medical Device UDI Submissions to the FDA GUDID PDF
EUDAMED Update What You Need to Know Now AssurX
UDI for Medical Devices Codes & Examples [Ultimate Guide]
Take Aim. It's UDI Season. Packaging Compliance Labs
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR)
UDI requirements for medical device manufacturers in the EU Medical
你想了解的UDI 欧盟UDI申请指南(二)
UDI Beginners Guide Unique Device Identification (EU MDR and IVDR)
PPT UDI From Regulation to Value PowerPoint Presentation, free
Related Post:
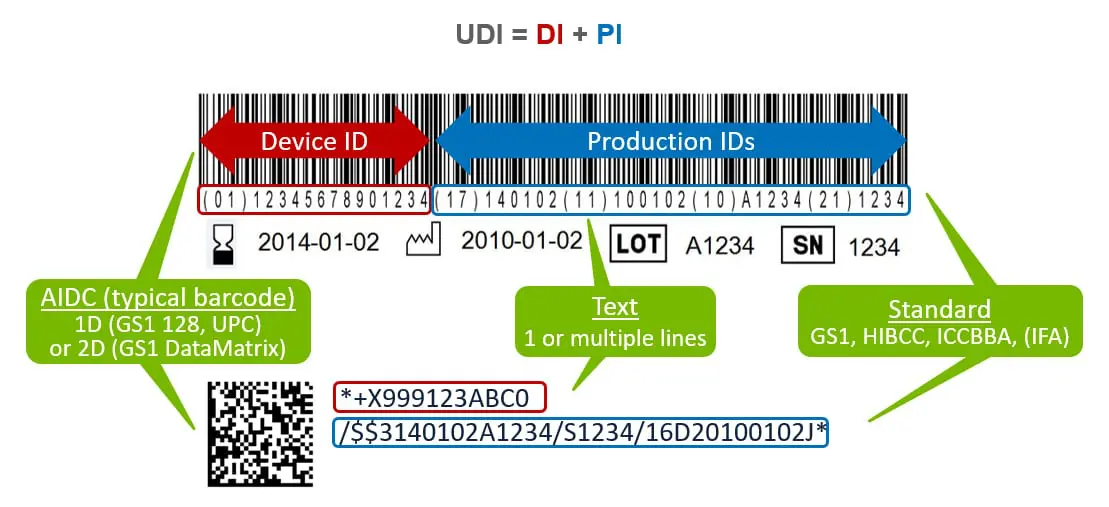
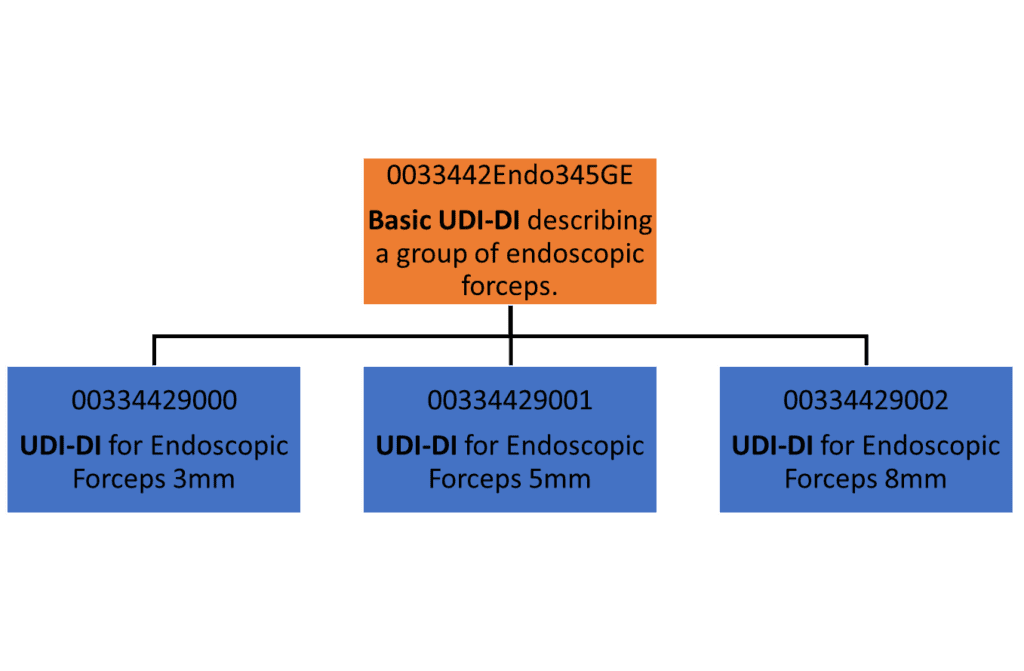
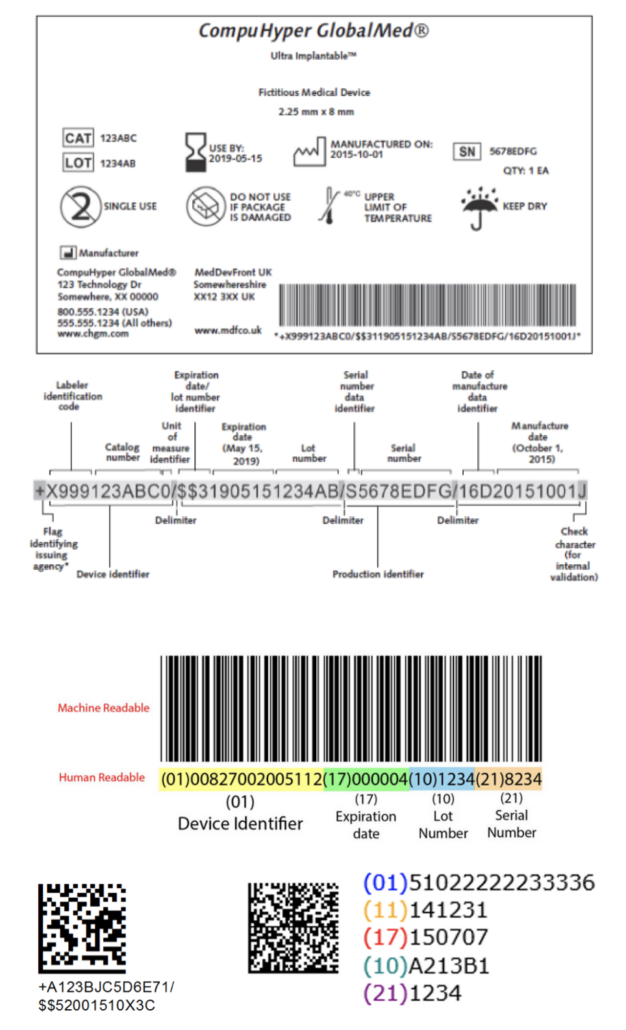
![UDI for Medical Devices Codes & Examples [Ultimate Guide]](https://www.greenlight.guru/hs-fs/hubfs/(cover) Ultimate-Guide-to-UDI.png?width=850&name=(cover) Ultimate-Guide-to-UDI.png)





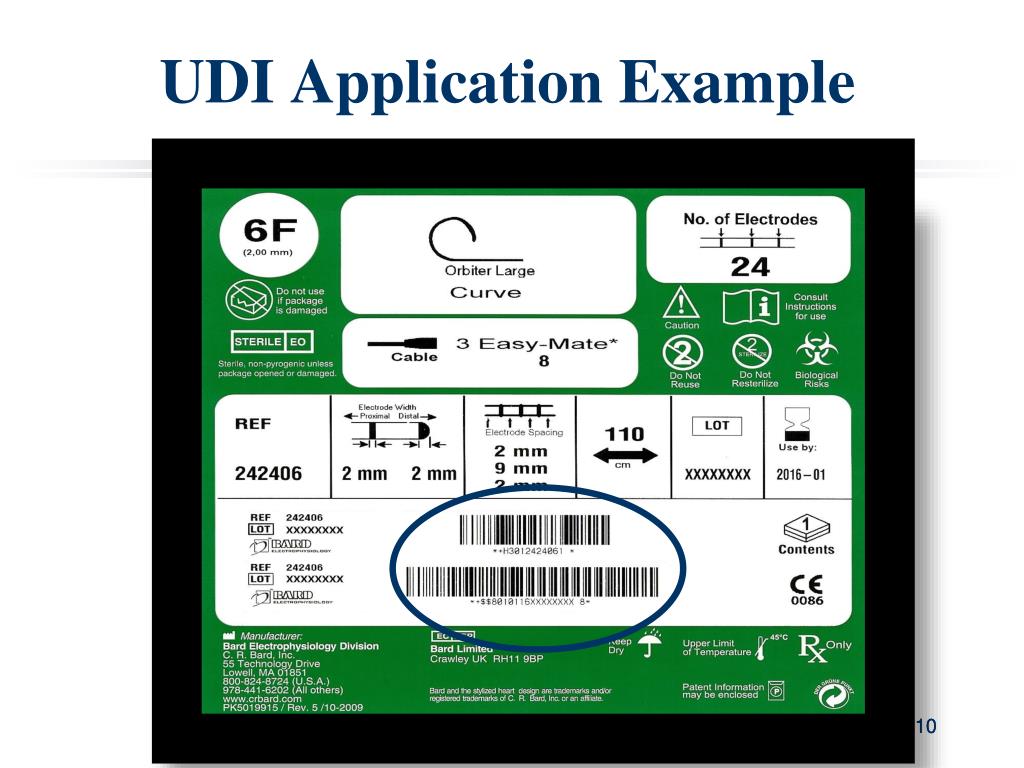
![UDI for Medical Devices Codes & Examples [Ultimate Guide]](https://www.greenlight.guru/hubfs/Ultimate Guide to UDI for Medical Devices-1.png#keepProtocol)

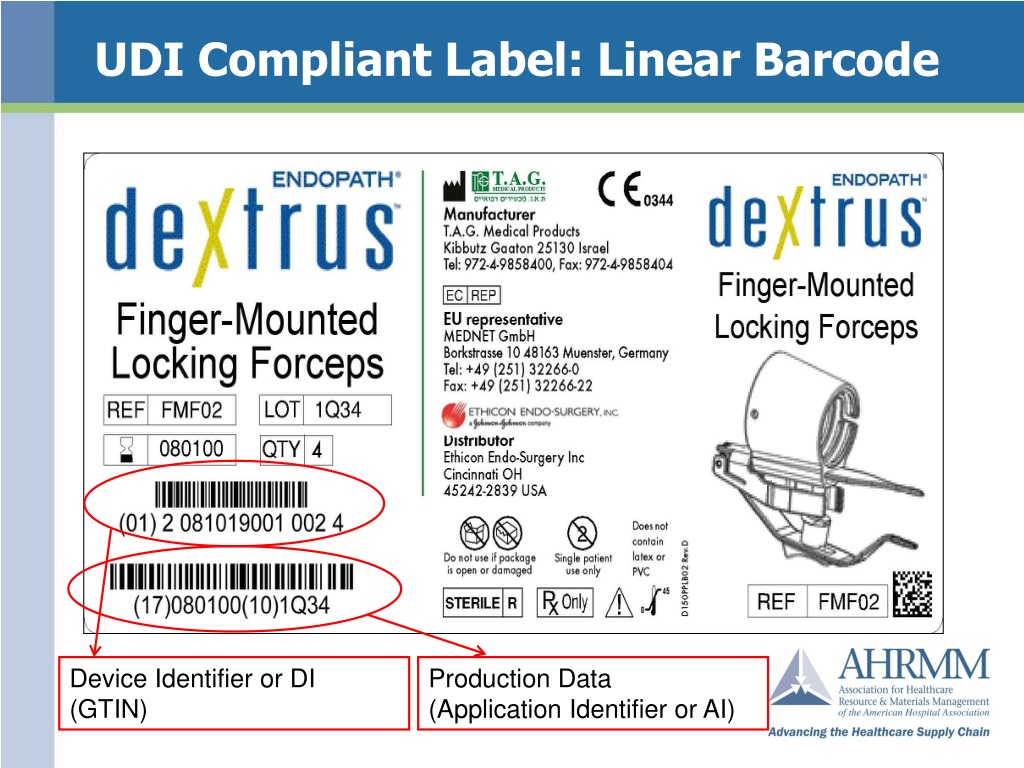
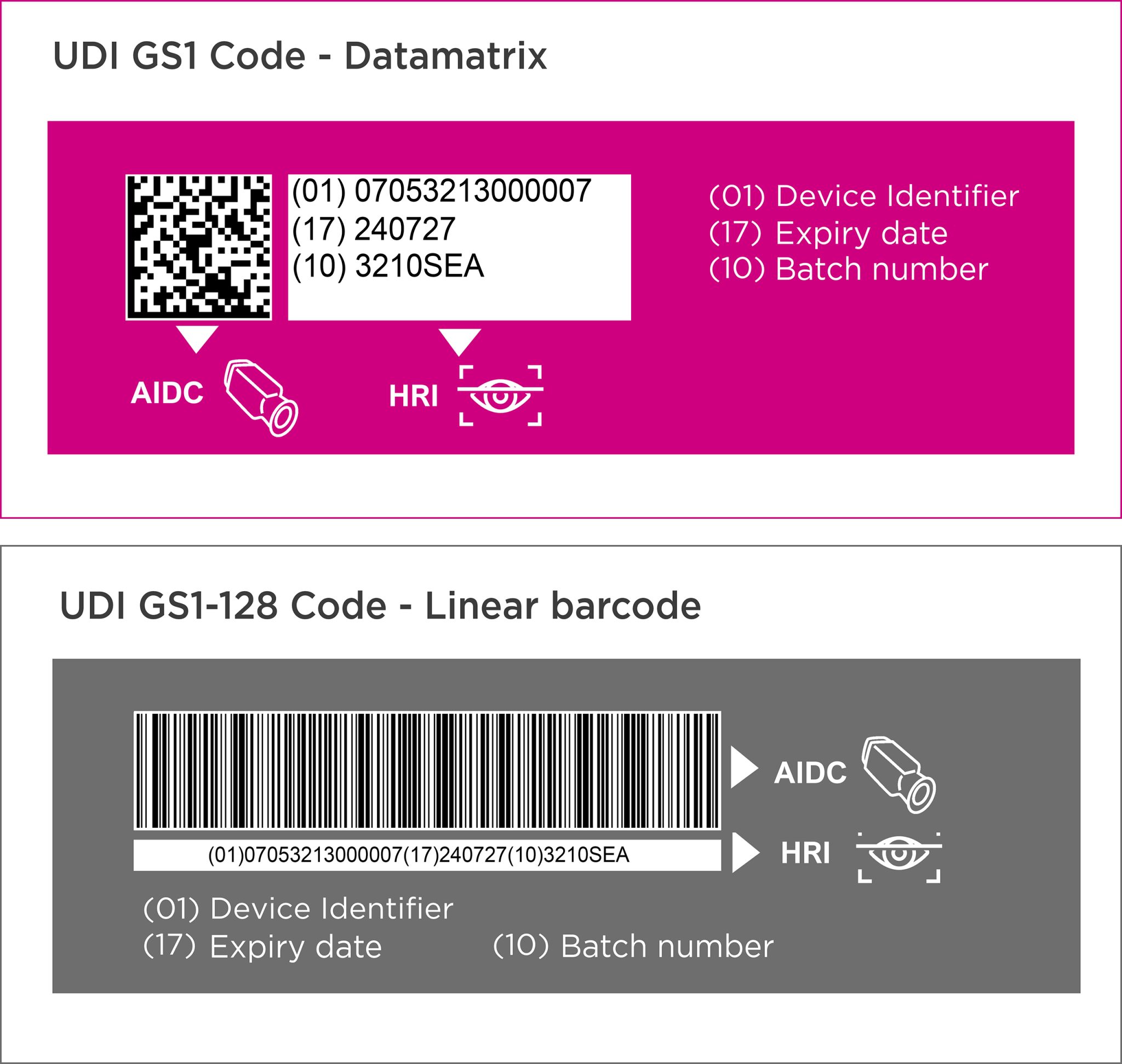

![UDI for Medical Devices Codes & Examples [Ultimate Guide]](https://blog.greenlight.guru/hubfs/EU_Chart-01.png)

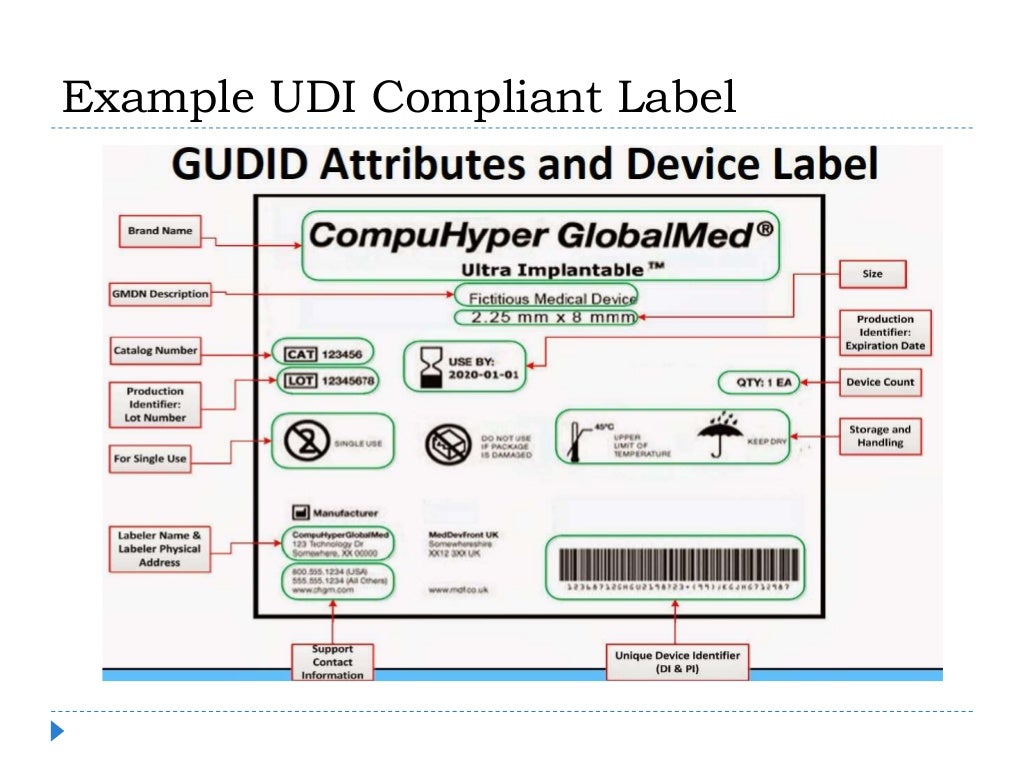

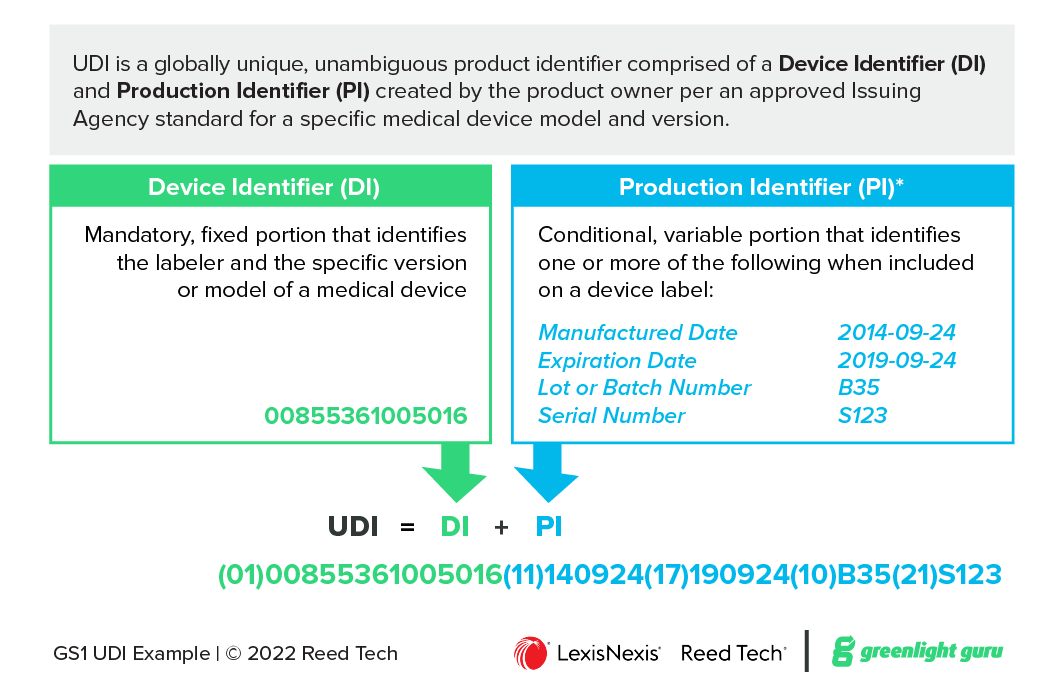


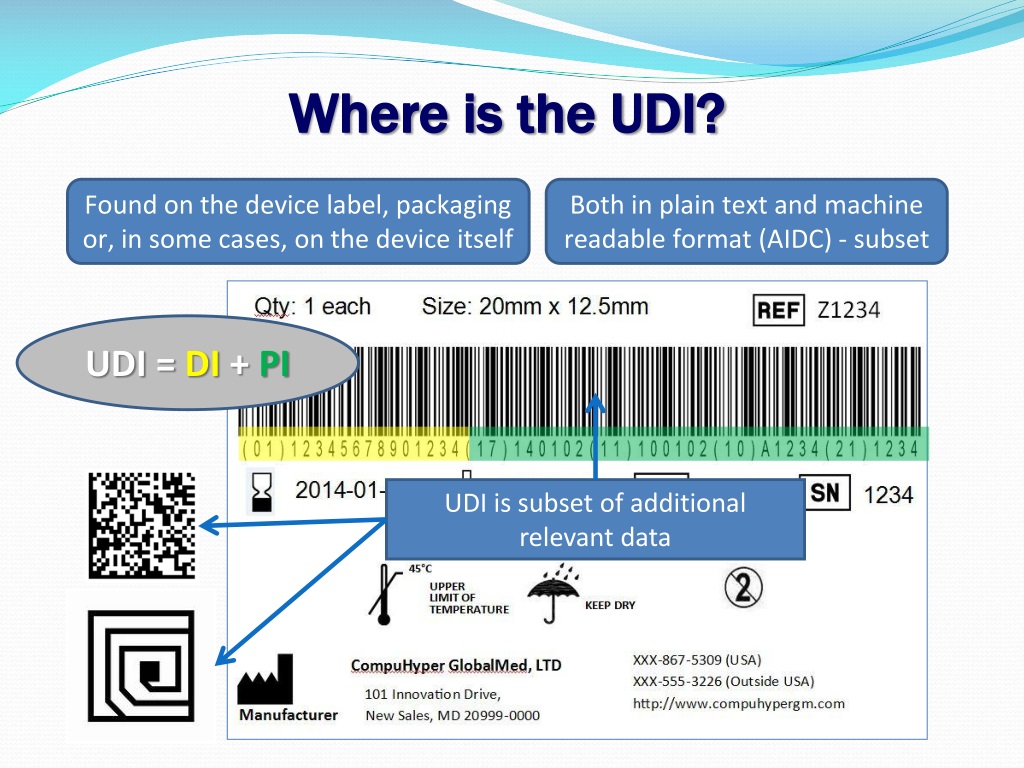
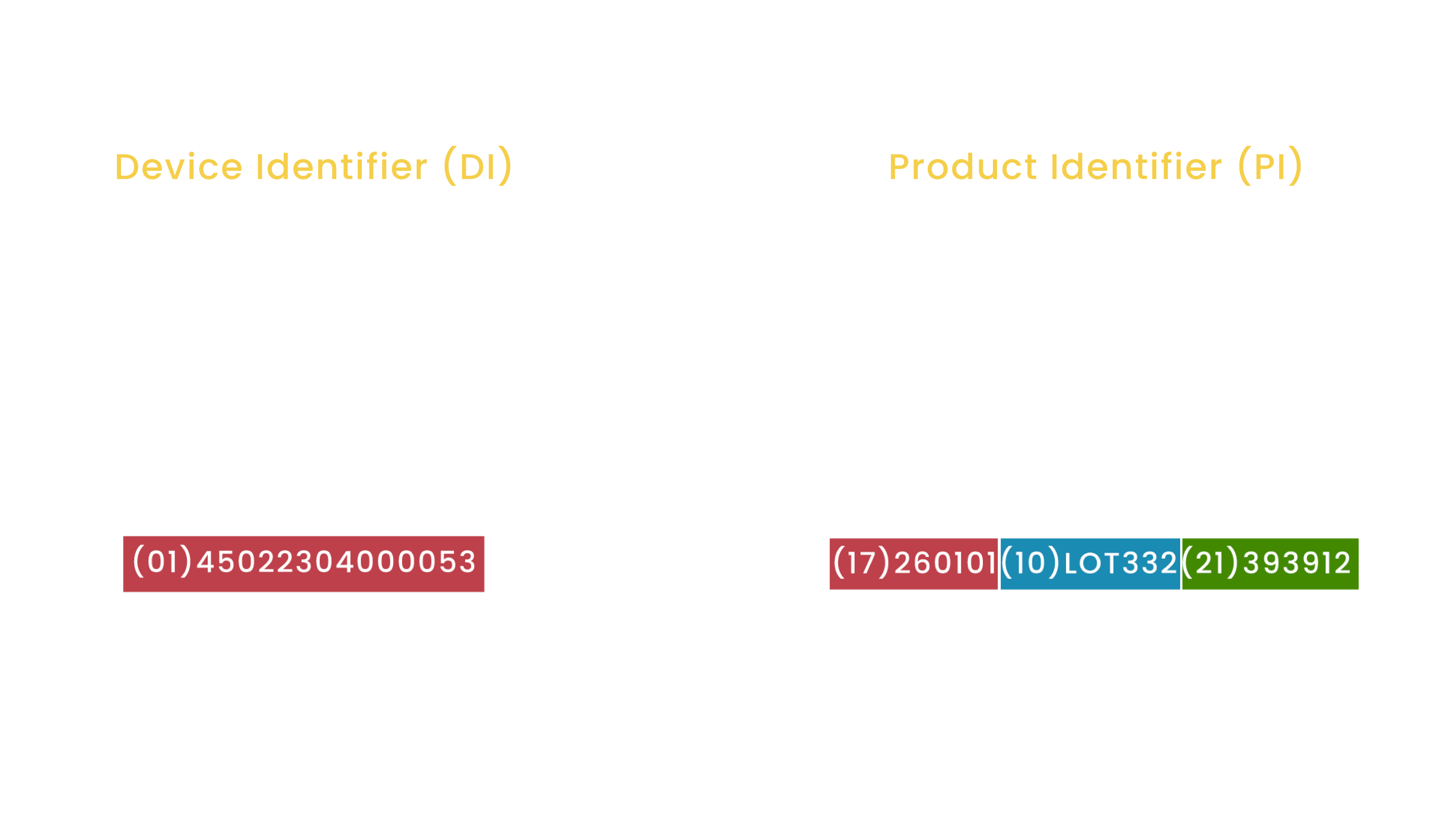


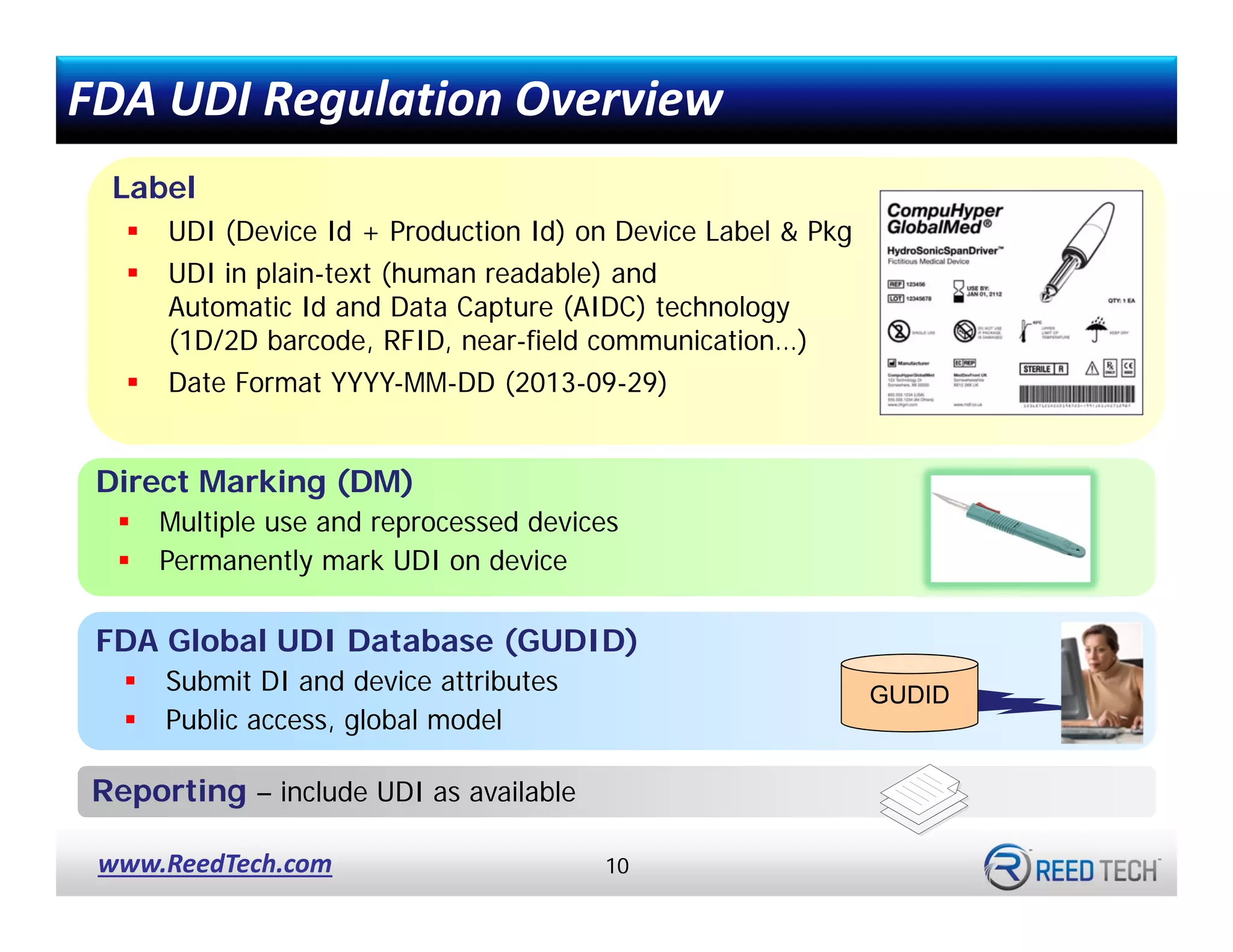
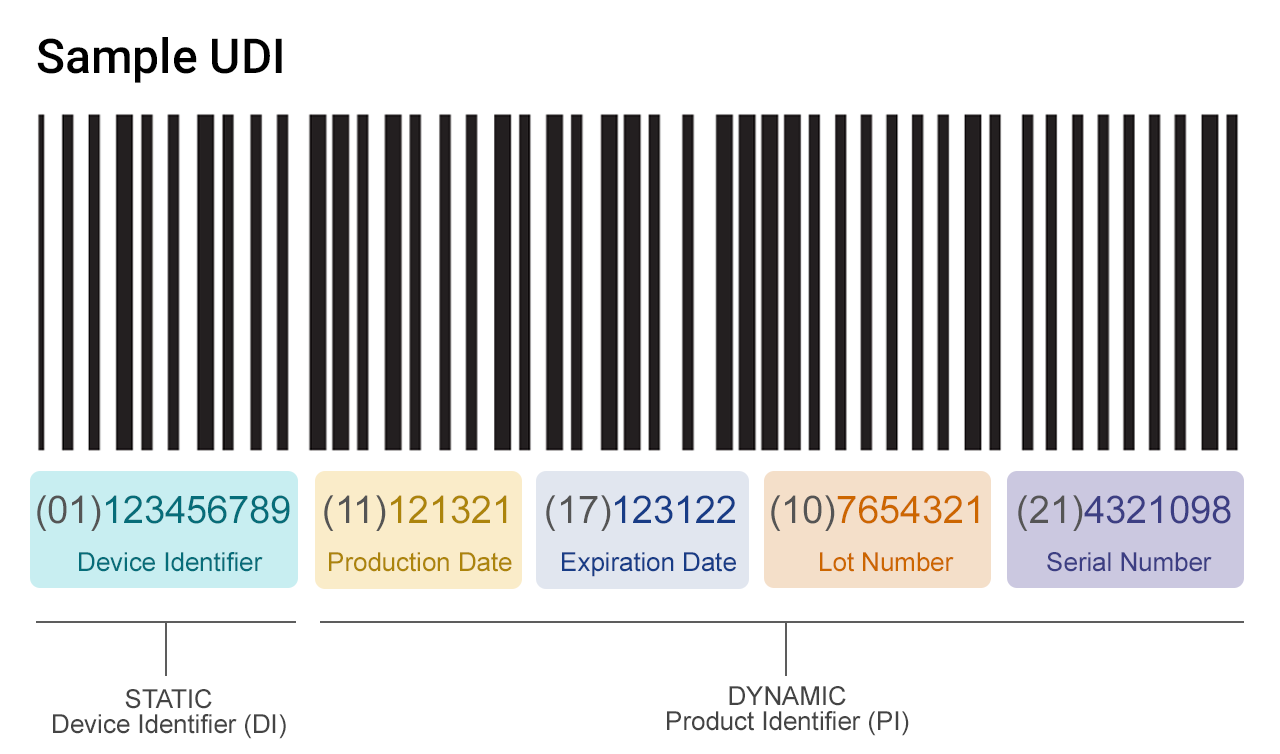
![UDI for Medical Devices Codes & Examples [Ultimate Guide]](https://blog.greenlight.guru/hubfs/FDA_Chart-01.png)